This website is designed to support you with your treatment and is not a substitute for the Package Leaflet that came with your medication. Information about Side Effects can be found on the Safety page. Instructions for reporting side effects are in the footer of this page.
Do not use Trokide®: If you are allergic to tiotropium, milk proteins or any of the other ingredients of this medicine (listed in the package leaflet) or if you are allergic to atropine or substances related to it, e.g., ipratropium or oxitropium.
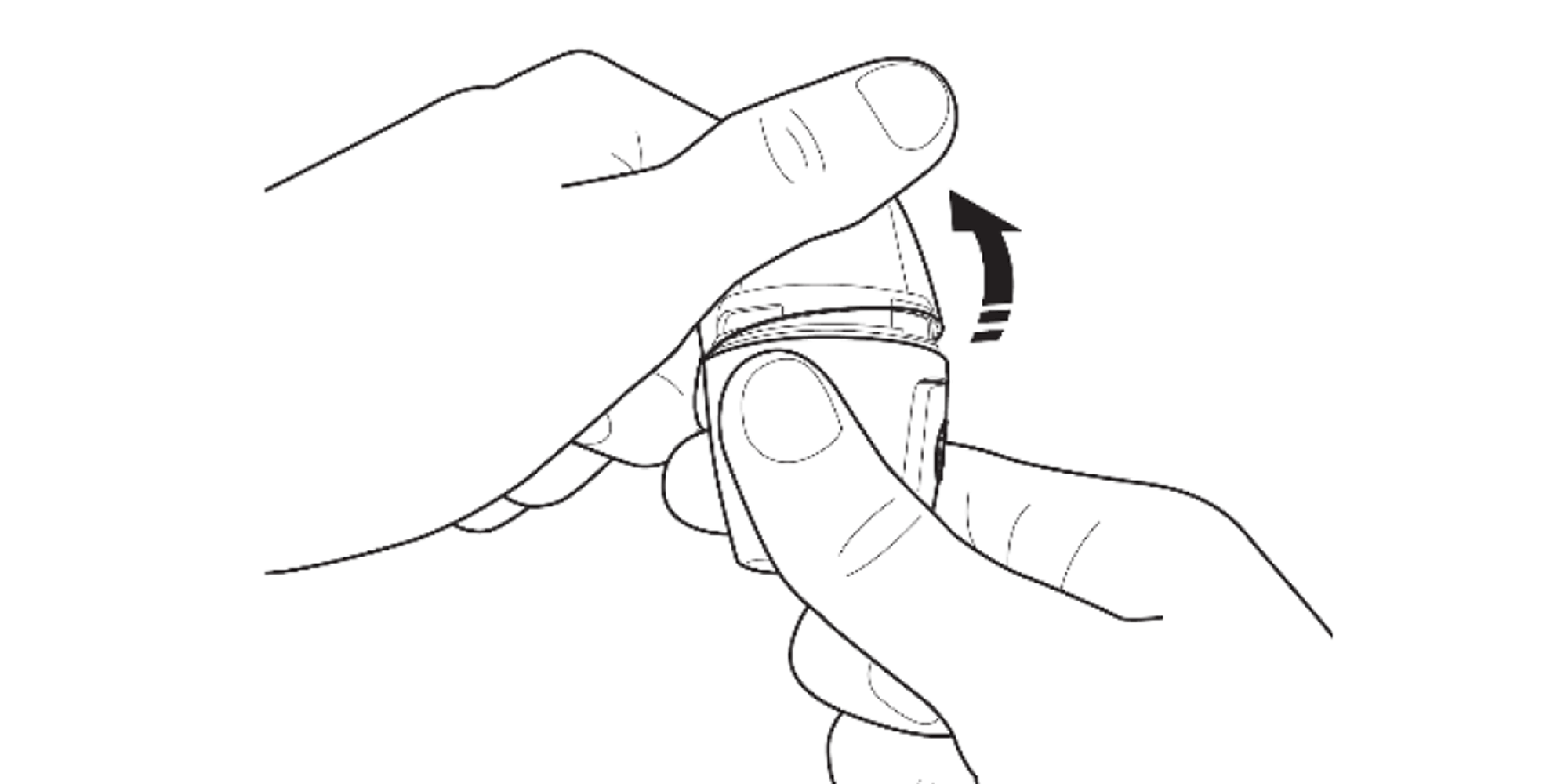
Remove the cap and hold the Vertical-Haler with the mouthpiece upwards.
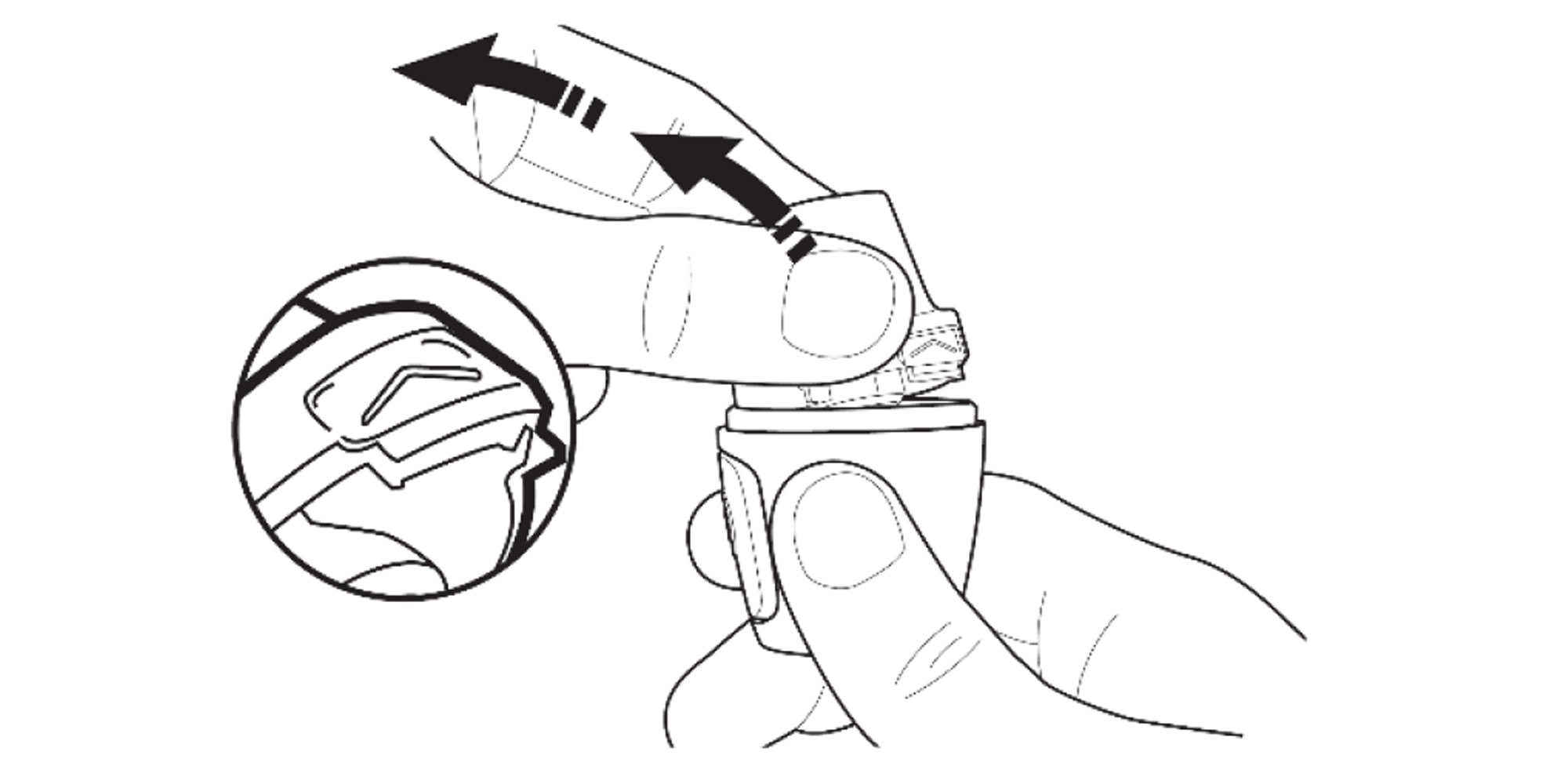
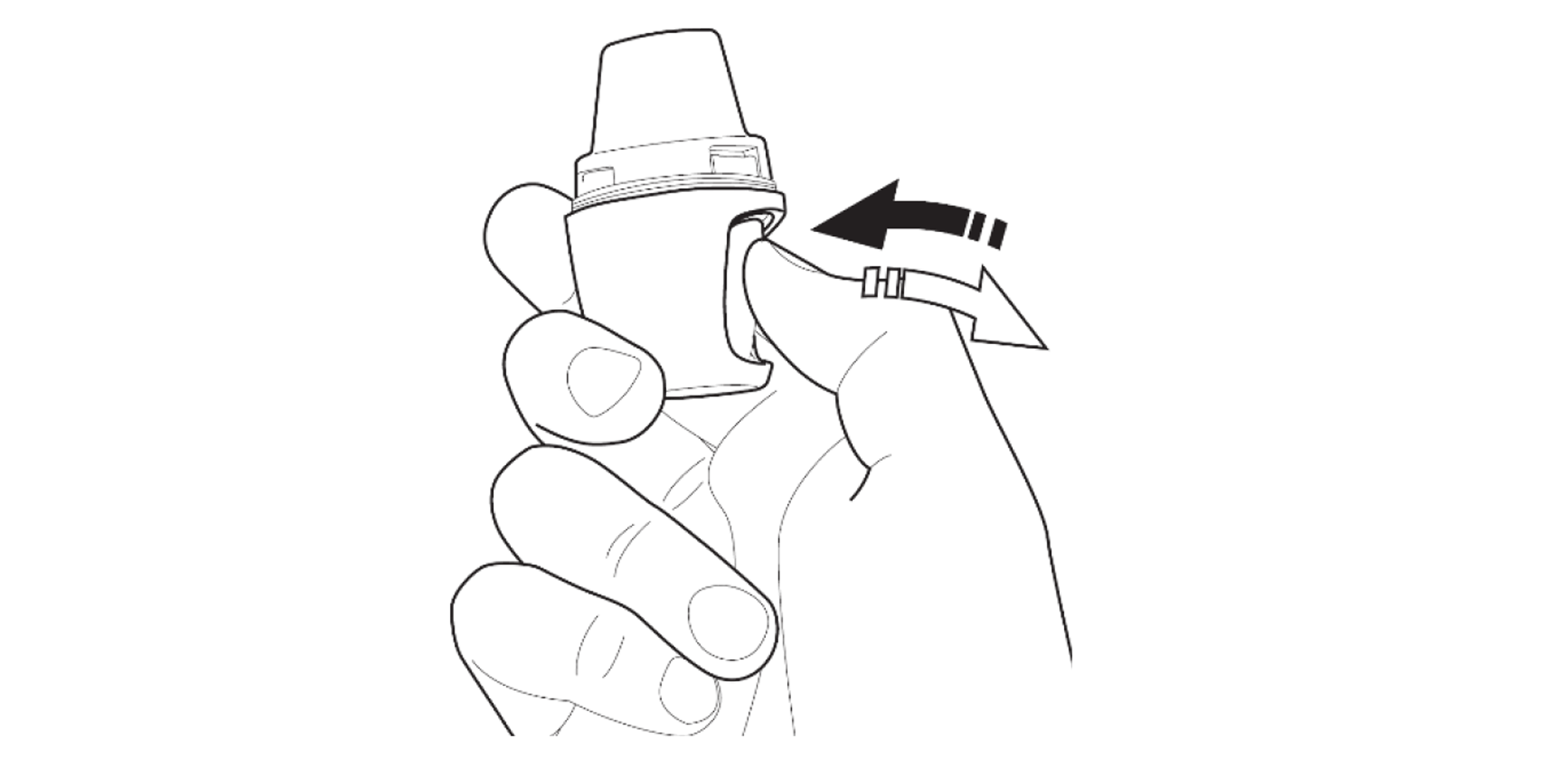
Press the area marked with a chevron. Pull the mouthpiece up and away from the base.
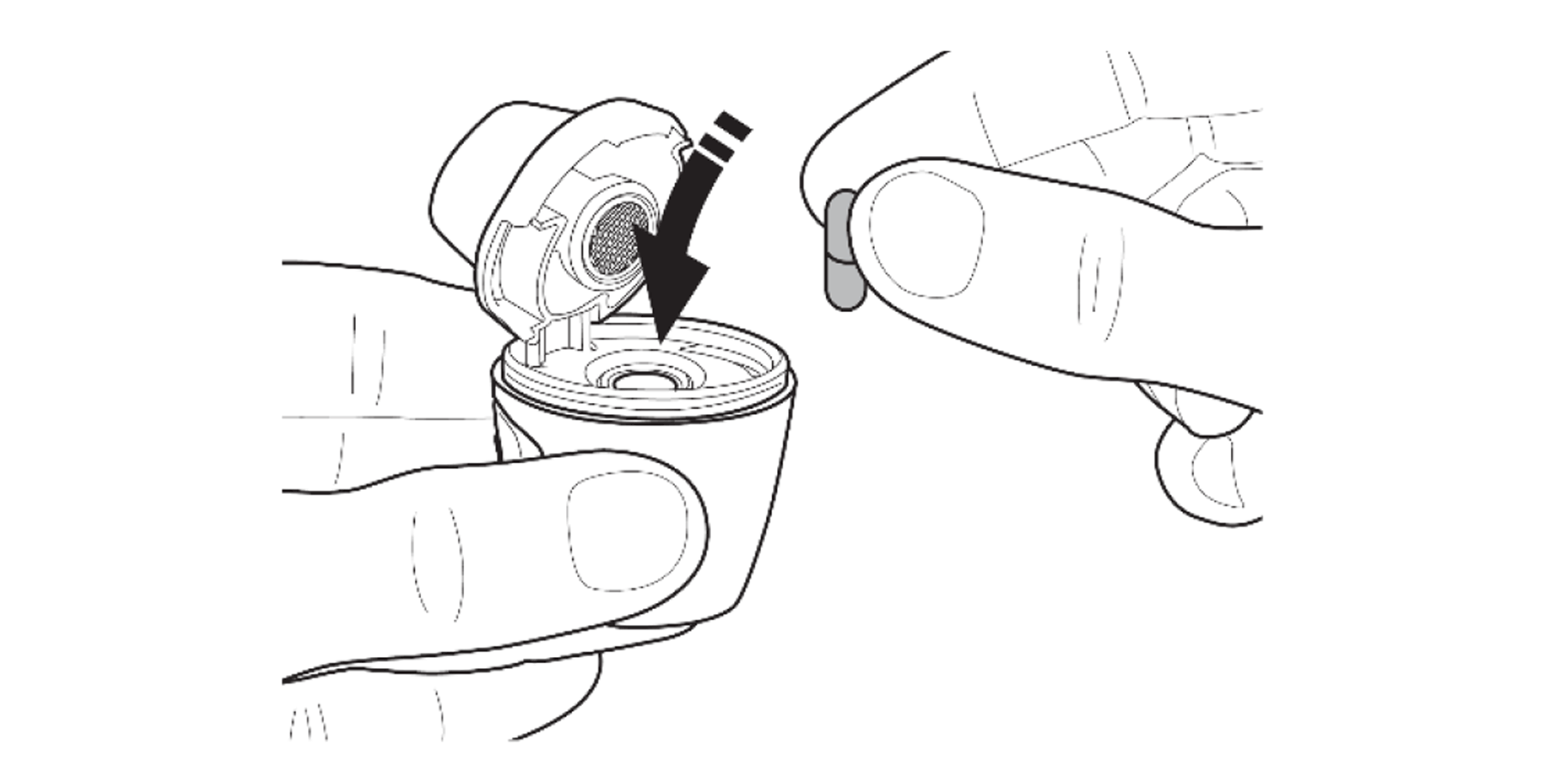
Remove the capsule from the blister and insert it into the capsule chamber.
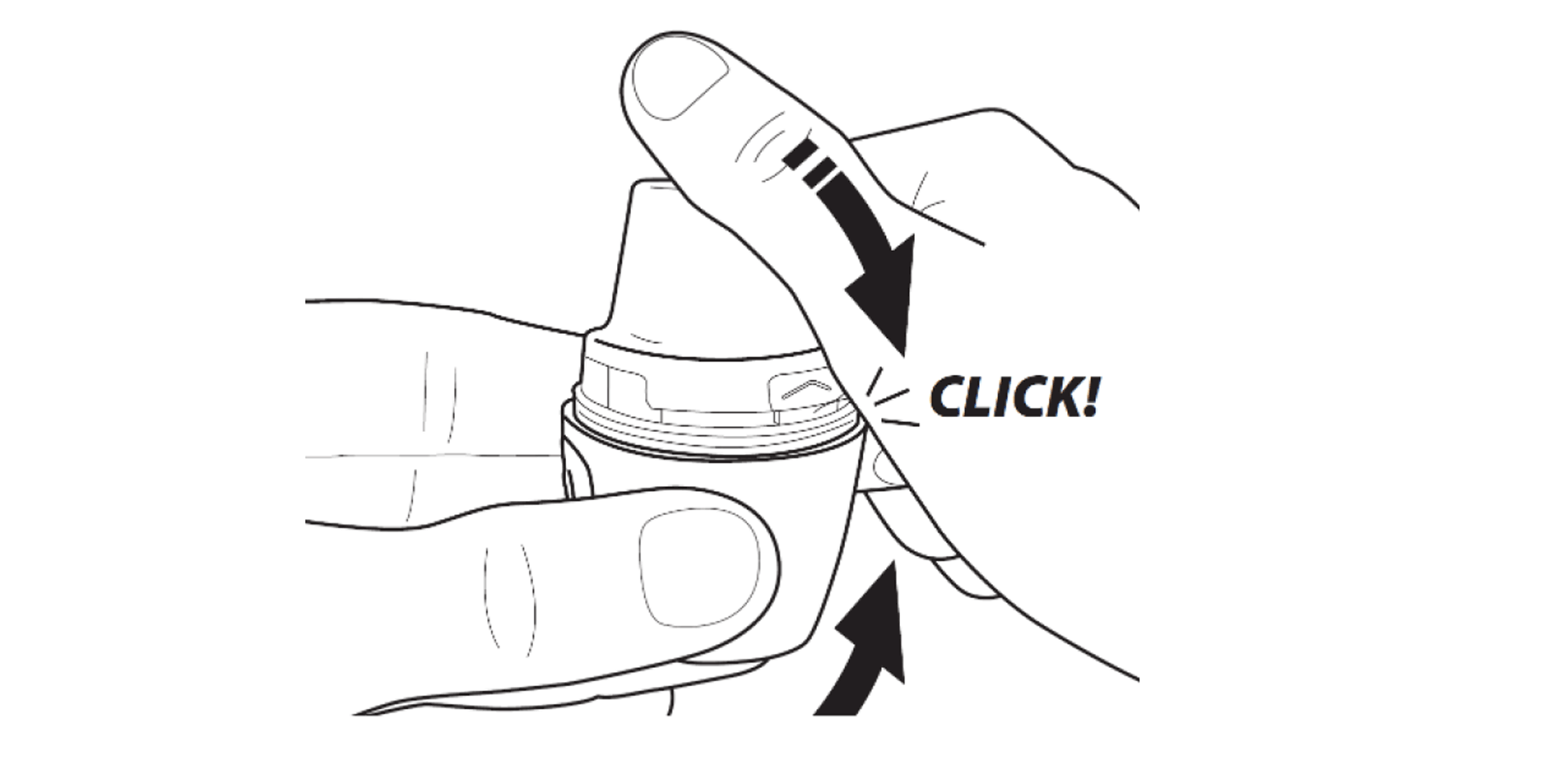
Firmly press the mouthpiece closed until you hear a click.
With the mouthpiece facing upwards, fully press the button in and release. Only press the button once!
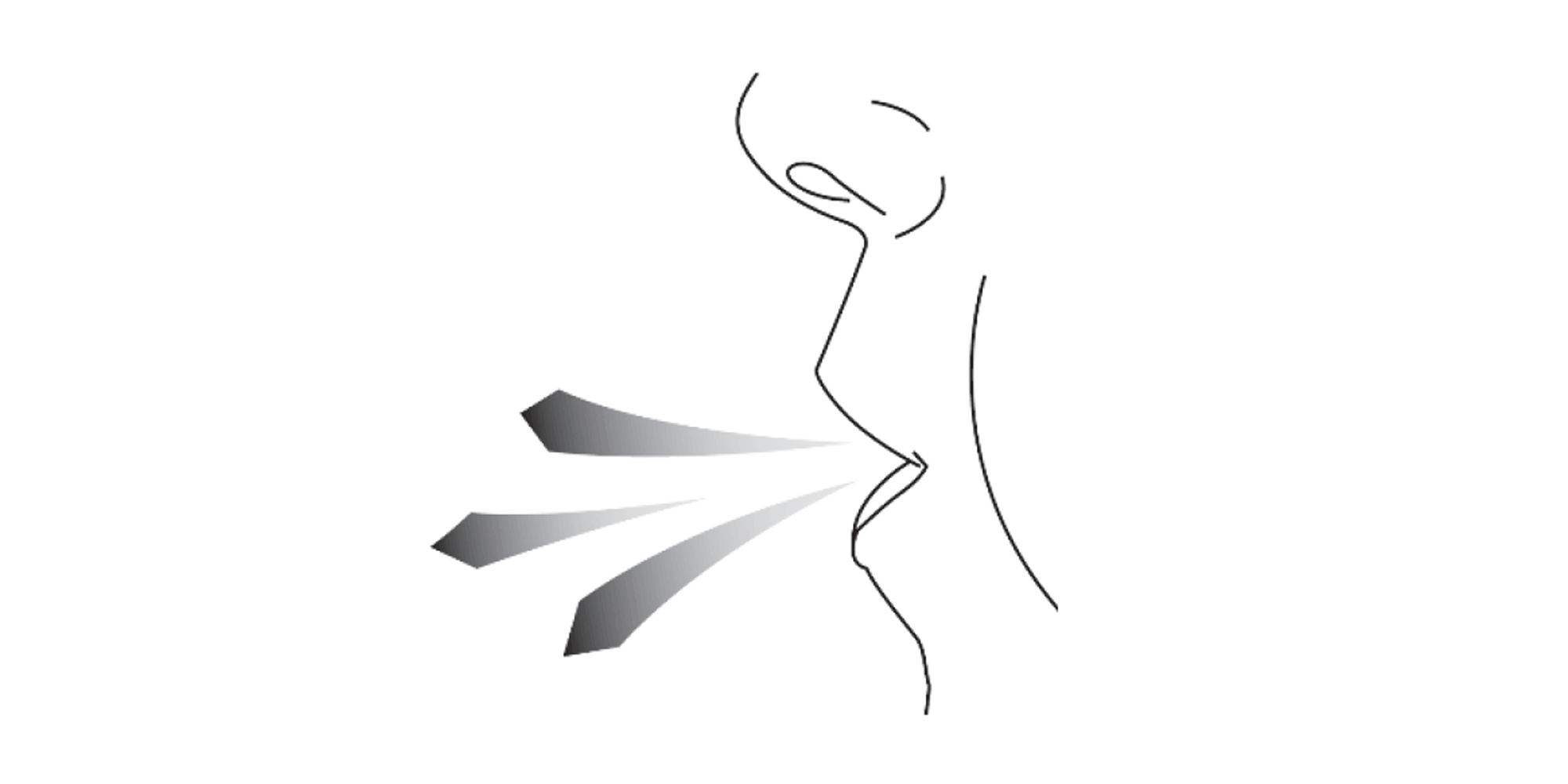
Hold the Vertical-Haler away from your mouth and breathe out completely. Do not breathe into the Vertical-Haler at any time.
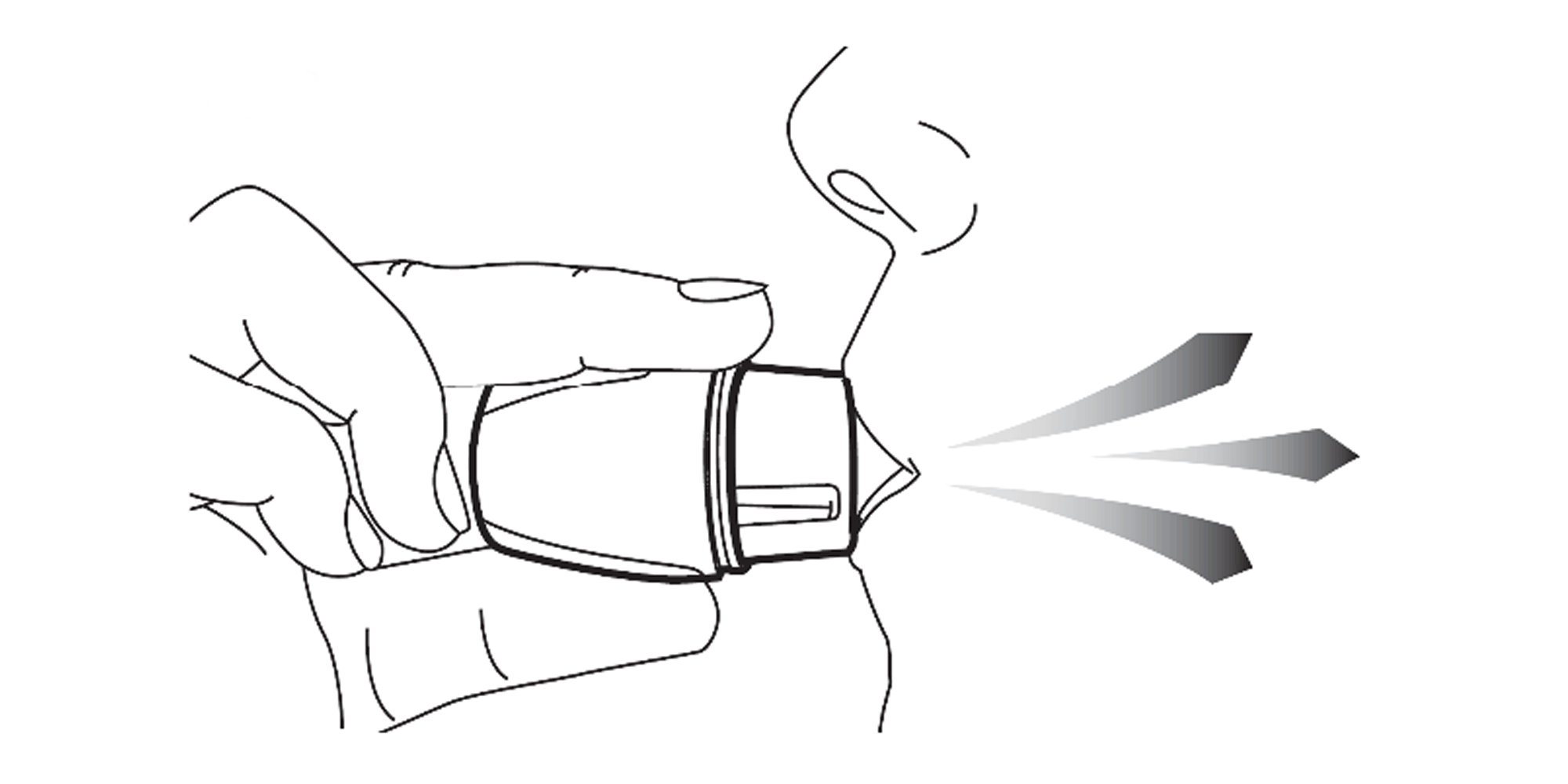
Keep your head in an upright position and close your lips tightly around the mouthpiece. Breathe in slowly and deeply until your lungs are full (it’s normal to hear a vibrating sound when breathing in). Take the Vertical-Haler out of your mouth and hold your breath for as long as is comfortable. Then breathe normally.
You should now repeat steps 2 and 3 as it is important to breathe in twice from the same capsule.
Always use this medicine exactly as your doctor, nurse or pharmacist has told you. Try to use your Vertical-Haler at the same time every day – this is because Trokide® is effective over 24 hours. Trokide® capsules must only be used with the Vertical-Haler, as it is especially designed for Trokide®. Do not block the air inlets or push the mouthpiece down during inhalation.
After use, open the mouthpiece. Tip the inhaler to remove the capsule from the capsule chamber. Close the mouthpiece and place the cap on. Discard your Vertical-Haler 90 days after first use. Ask your doctor, nurse or pharmacist if you require a new one.
UK-TROK-27b | December 2024